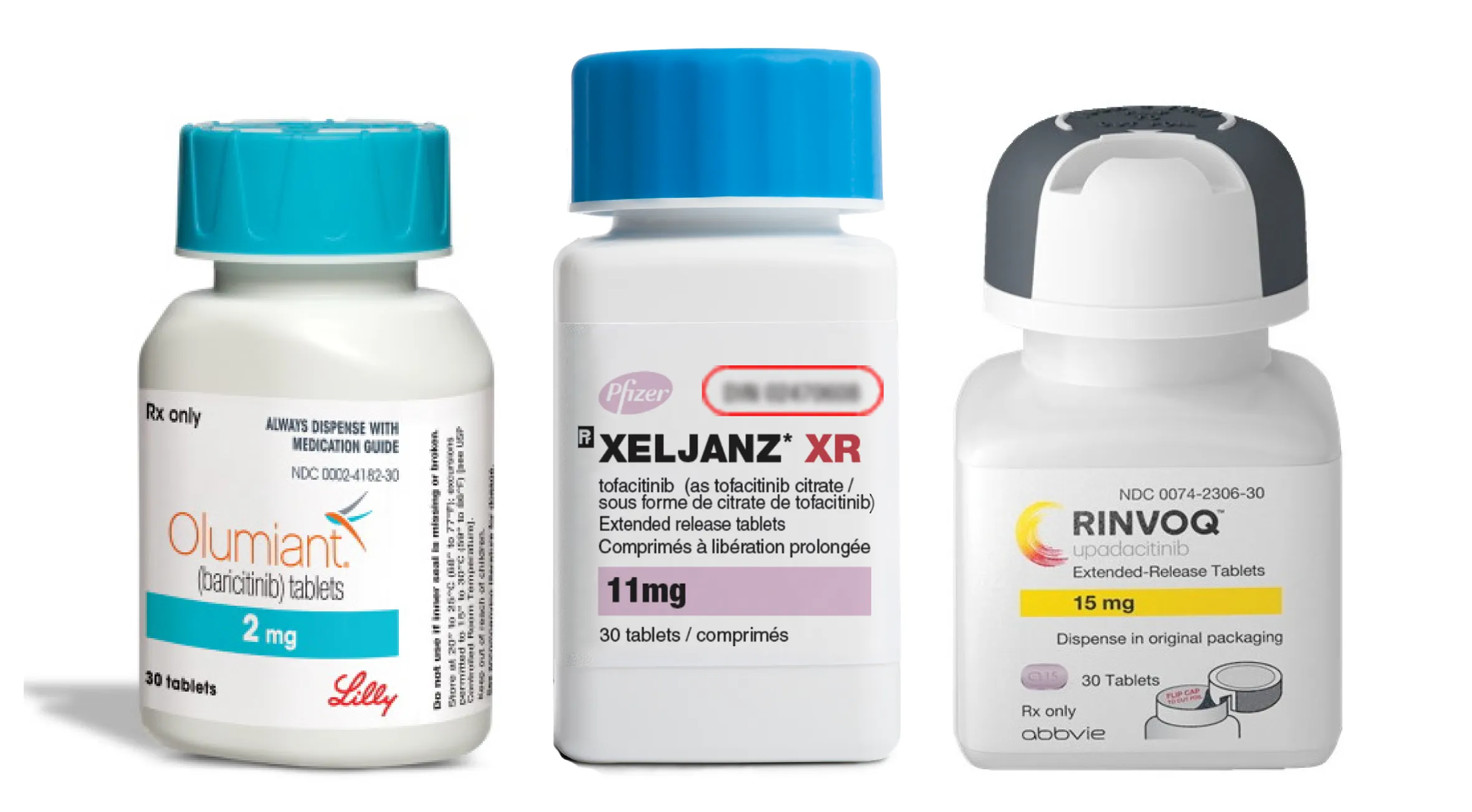
If you’re anything like me, your Hulu commercials have been full of advertisements for drugs like Rinvoq, Olumiant, or Xeljanz. These medications all belong to a class of drugs called Janus Kinase Inhibitors, or JAK inhibitors. Recently, many of these medications have been FDA approved for different dermatological conditions. There are also several ongoing clinical trials using these medications for a whole bunch of inflammatory skin conditions, many with positive results. So what are these medications, how do they work, and why are they the next big thing in Dermatology?
What are JAK inhibitors?
JAK inhibitors are a class of medications that work on a cellular signaling pathway called the JAK-STAT pathway. This pathway receives input from your body and tells your cells to make more cytokines, or cellular messengers, that increase inflammation in the body. This underlying inflammation is what is responsible for many skin, hair, and nail conditions. JAK inhibitors help prevent these cytokines from being produced, thereby improving diseases such as alopecia areata, vitiligo, and eczema. JAK inhibitors were first studied and used to treat rheumatoid arthritis.
What can they treat?
JAK inhibitors are currently FDA-approved to treat eczema (Opzelura, Cibinqo, Rinvoq), alopecia areata (Olumiant, Leqselvi, Litfulo), psoriatic arthritis (Xeljanz, Rinvoq), and vitiligo (Opzelura). They are all oral medications, with the exception of Opzelura, which is a cream. There are case reports and clinical studies demonstrating that JAK inhibitors can be used off-label to treat a variety of dermatological conditions such as scarring alopecia, bullous pemphigoid, dermatomyositis, graft-versus-host disease, and hidradenitis suppurativa. This promising class of medications has the potential to change the field of Dermatology!
What do I need to know?
The benefits of these medications are that they are oral medications (not an injection), and often work for patients who have failed many other treatments. JAK inhibitors are immune-modifying; therefore, they can suppress the immune system. This means there is an increased risk of infection while taking them. Additionally, you may have heard of something called a “Black Box Warning” on this class of medications. This was placed on all JAK inhibitors after post-market analysis of JAK inhibitors for rheumatoid arthritis patients in which they found a higher incidence of malignancy, thrombosis, and major cardiovascular events. However, experts have pointed out that people with rheumatoid arthritis are already more at-risk for these conditions, which may have skewed the data. More recent studies have found that the risk of these adverse events is lower in younger, healthy individuals. Other common side effects of JAK inhibitors include acne, upper respiratory infection, nausea, and headache.
Your board-certified dermatologist will be able to tell you if a JAK inhibitor is a good option for you based on your overall health and medical history. If you do decide to start a JAK inhibitor, your dermatologist will get baseline blood tests, a HIV test, a tuberculosis test, and a hepatitis test. You may then be monitored with blood tests for liver and kidney function and cholesterol after starting the medication.