If you or a loved one has been diagnosed with cancer and are facing a long journey of treatment, the prospect of hair loss and the visible nature of the disease can add an extra layer of emotional strain, intensifying feelings of vulnerability and loss of privacy. Chemotherapy-induced alopecia is one of the dreaded side effects of chemotherapy which has been shown to cause some patients to avoid treatment altogether. Certain chemotherapy regimens– especially those used to treat gynecologic and breast cancers– are particularly noted to cause hair loss. In an effort to combat this side-effect, scalp cooling methods have been employed. Scalp cooling is an FDA-approved method for the prevention of chemotherapy-induced alopecia.
What is Chemotherapy-Induced Alopecia?
Chemotherapy-induced alopecia (CIA) is hair loss caused by certain chemotherapeutic agents, including Doxorubicin, Paclitaxel, and Cyclophosphamide to name a few. It typically presents as diffuse hair loss within two weeks of starting treatment. Chemotherapy works by targeting the rapidly dividing cells of the cancer, however, most treatment is non-specific, and the body’s normal rapidly dividing cells are also affected. Therefore, cells within the hair follicle matrix are impacted causing rapid shedding of the hair. Though CIA is temporary in most people, some individuals experience permanent alterations to their hair color, texture, and density.
How does scalp cooling work?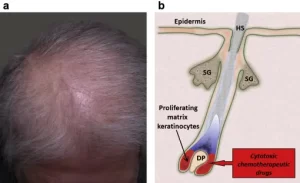
Scalp cooling is thought to work through several pathways. By subjecting the scalp to cold temperatures, the blood vessels get smaller and less blood makes its way to the hair follicles. The cold also reduces the hair cells’ metabolic activity. Overall, this decreases the amount of chemotherapy drug that reaches the hair follicle, which helps the hairs survive. There are currently three FDA-approved devices: Penguin Cold Caps, DigniCap Scalp Cooling System, and Paxman Scalp Cooling System. There are also manual cold caps, but these are not regulated by the FDA.
The process for scalp cooling is as follows: thirty-minutes prior to chemotherapy, a cold cap is placed on the patient’s head. The cap is hooked up to a machine that will continuously circulate refrigerant through the cap, keeping the scalp between 59-77 degrees Fahrenheit. The cap stays on throughout the chemotherapy treatment and for 90-120 minutes afterwards while the drug is still at a high concentration in the body.
Does it actually work?
There have been several clinical trials looking at the success of scalp cooling in preventing CIA. A recent meta-analysis of 8 clinical trials found that there was a statistically significant reduction in the risk of CIA by 47% in those who used automated scalp cooling devices. When reading research studies, it is important to note that many of them report “scalp cooling success” as patients retaining some or most of their hair (>50%). Many patients still lose some hair during the process, which is expected!
Of note, several patient factors can impact the efficacy of scalp cooling. For example, hair type can impact the cap’s ability to contact the skin properly. Individuals with kinky/curly hair may have to braid or wet their hair in order to wear the cap effectively. There are currently clinical trials working on finding the best methods for scalp cooling in people with this hair type. Further, scalp cooling success also depends on the type of chemotherapy utilized.
What are the side effects?
The reported side effects of scalp cooling include headache, nausea, dry skin, and general discomfort from being cold. It is generally very safe and well-tolerated. Research studies have shown that scalp cooling does not appear to increase the incidence of scalp metastasis.
Is it covered by insurance?
Unfortunately, insurance coverage for scalp cooling is not standard in the United States. Some insurances, including Medicaid, may cover part of this treatment. Scalp cooling costs on average $1,500 to $3,000 out-of-pocket depending on the number of chemotherapy cycles needed.
Below are some resources for more information, research studies, and finding cancer centers that use scalp cooling technology. It is important to know that scalp cooling cannot be used with all types of chemotherapy regimens or in all types of cancer. As always, if you are considering scalp cooling, talk to your doctors to see if it may be an option for you!
https://www.cancer.org/cancer/managing-cancer/side-effects/hair-skin-nails/hair-loss/cold-caps.html
https://scalpcoolingstudies.com
http://www.rapunzelproject.org/ColdCaps/Locations.aspx
https://coldcap.com/scalp-cooling-system-locator/
https://clinicaltrials.gov/search?intr=Scalp%20cooling